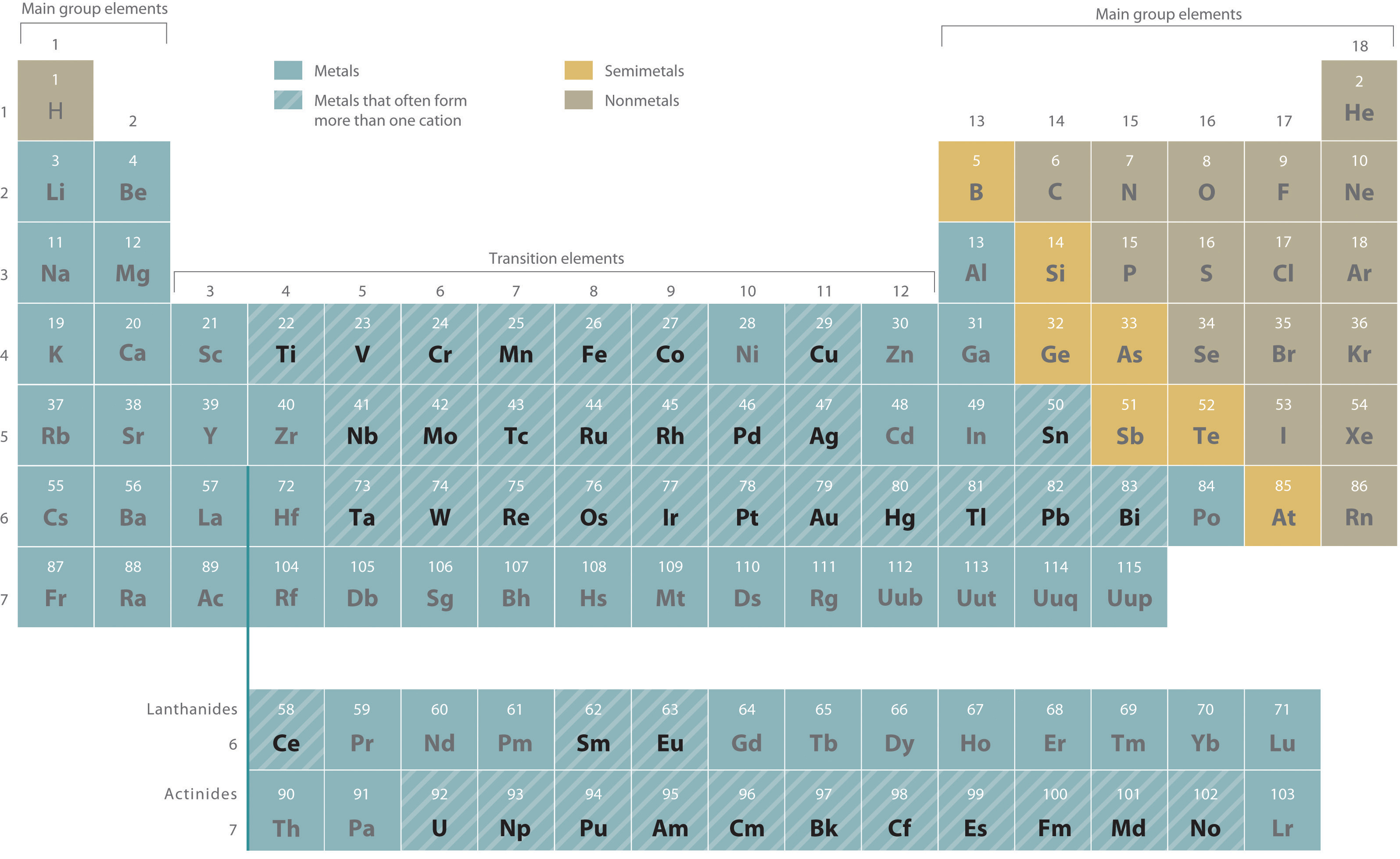
Are Ionic Compounds Metals . A compound that contains ions and is held together by ionic bonds is called an ionic compound. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: The periodic table can help us. Ionic compounds usually dissociate in water because water is a polar molecule. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds contain positively and negatively charged ions in a ratio that results in an overall charge of zero. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. The o atom in water. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the atoms together. The ions are held together in a.
from saylordotorg.github.io
Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic compounds contain positively and negatively charged ions in a ratio that results in an overall charge of zero. The periodic table can help us. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. The ions are held together in a. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the atoms together. A compound that contains ions and is held together by ionic bonds is called an ionic compound. The o atom in water. Ionic compounds usually dissociate in water because water is a polar molecule.
Naming Ionic Compounds
Are Ionic Compounds Metals The ions are held together in a. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. The o atom in water. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic compounds usually dissociate in water because water is a polar molecule. Ionic compounds contain positively and negatively charged ions in a ratio that results in an overall charge of zero. The ions are held together in a. A compound that contains ions and is held together by ionic bonds is called an ionic compound. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the atoms together. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. The periodic table can help us.
From www.youtube.com
Ionic Compounds and Bonding Part 06 Transition Metal Nomenclature Are Ionic Compounds Metals Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the atoms together. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. The periodic table can help us. The o atom in water. A compound that contains ions and is held together. Are Ionic Compounds Metals.
From www.slideserve.com
PPT Naming Chemical Compounds PowerPoint Presentation, free download Are Ionic Compounds Metals The periodic table can help us. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds usually dissociate in water because water is a polar molecule. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: The o atom in water. Molecular compounds. Are Ionic Compounds Metals.
From www.slideserve.com
PPT Ionic Compounds PowerPoint Presentation, free download ID3730496 Are Ionic Compounds Metals Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: The o atom in water. A compound that contains ions and is held together by ionic bonds is called an ionic compound. The ions are held together in a. Ionic compounds usually dissociate in water because water is a polar. Are Ionic Compounds Metals.
From www.slideserve.com
PPT Ionic vs. Molecular Compounds PowerPoint Presentation, free Are Ionic Compounds Metals Ionic compounds usually dissociate in water because water is a polar molecule. The ions are held together in a. The periodic table can help us. Ionic compounds contain positively and negatively charged ions in a ratio that results in an overall charge of zero. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. The. Are Ionic Compounds Metals.
From www.wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry Are Ionic Compounds Metals A compound that contains ions and is held together by ionic bonds is called an ionic compound. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Molecular compounds form between nonmetals and nonmetals, while. Are Ionic Compounds Metals.
From www.slideserve.com
PPT Molecular Compounds PowerPoint Presentation, free download ID Are Ionic Compounds Metals Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the atoms together. Ionic compounds contain positively and negatively charged ions in a ratio that results in an overall charge of zero. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. The. Are Ionic Compounds Metals.
From quizizz.com
Properties of Ionic Compounds Chemistry Quiz Quizizz Are Ionic Compounds Metals Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. Ionic compounds usually dissociate in water because water is a polar molecule. The ions are held together in a. Solutions of ionic compounds and melted. Are Ionic Compounds Metals.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Are Ionic Compounds Metals Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the atoms together. Ionic compounds usually dissociate in water because water is a polar molecule. The o atom in water. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. A compound that. Are Ionic Compounds Metals.
From jaylenyouthmejia.blogspot.com
Ionic Bonding Between Metals and Nonmetals Are Ionic Compounds Metals The ions are held together in a. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. The o atom in water. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the atoms together. Ionic compounds contain positively and negatively charged ions. Are Ionic Compounds Metals.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Are Ionic Compounds Metals Ionic compounds contain positively and negatively charged ions in a ratio that results in an overall charge of zero. The ions are held together in a. The o atom in water. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions. Are Ionic Compounds Metals.
From www.slideserve.com
PPT Writing and Naming Ionic compounds (criss cross method Are Ionic Compounds Metals A compound that contains ions and is held together by ionic bonds is called an ionic compound. Ionic compounds contain positively and negatively charged ions in a ratio that results in an overall charge of zero. Ionic compounds usually dissociate in water because water is a polar molecule. Compounds composed of ions are called ionic compounds (or salts), and their. Are Ionic Compounds Metals.
From www.youtube.com
Transition Metal Ionic Compound Names and Formulas YouTube Are Ionic Compounds Metals Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds contain positively and negatively charged ions in a ratio that results in an overall charge of zero. A compound that contains ions and is held together by ionic bonds is called an ionic compound. The periodic table can help us. Ionic compound, any. Are Ionic Compounds Metals.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Are Ionic Compounds Metals The o atom in water. The ions are held together in a. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the atoms together. A compound that contains ions and is held together by ionic bonds is called an ionic compound. Ionic compounds usually dissociate in water. Are Ionic Compounds Metals.
From saylordotorg.github.io
Naming Ionic Compounds Are Ionic Compounds Metals Ionic compounds usually dissociate in water because water is a polar molecule. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. The periodic table can help us. Ionic compounds contain positively and negatively charged. Are Ionic Compounds Metals.
From www.slideserve.com
PPT Nomenclature PowerPoint Presentation, free download ID4848961 Are Ionic Compounds Metals A compound that contains ions and is held together by ionic bonds is called an ionic compound. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. The periodic table can help us. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic compounds. Are Ionic Compounds Metals.
From www.animalia-life.club
Ionic Compounds Periodic Table Are Ionic Compounds Metals Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds contain positively and negatively charged ions in a ratio that results in an overall charge of zero. The periodic table can help us. The ions are held together in a. Compounds composed of ions are called ionic compounds (or salts), and their constituent. Are Ionic Compounds Metals.
From www.breakingatom.com
Ionic Bonding Are Ionic Compounds Metals The o atom in water. Ionic compounds usually dissociate in water because water is a polar molecule. A compound that contains ions and is held together by ionic bonds is called an ionic compound. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. Solutions of ionic compounds and melted ionic compounds conduct electricity, but. Are Ionic Compounds Metals.
From www.slideserve.com
PPT Names and Formulas Ionic Compounds Binary Main Group M & NM Using Are Ionic Compounds Metals Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: The ions are held together in a. Ionic compounds contain positively and negatively charged ions in a ratio that results in an overall charge of zero. Ionic compounds usually dissociate in water because water is a polar molecule. Molecular compounds. Are Ionic Compounds Metals.